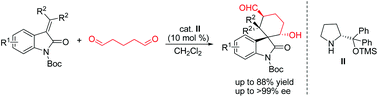
Optically active spirocyclohexaneoxindole motifs are very important building blocks for preparations of biologically active complexes, natural products, and pharmaceutical compounds. Herein, we report the syntheses of enantiopure spirocyclohexaneoxindoles through domino Michael–Aldol reactions between isatin derived alkenes and pentane-1,5-dial in the presence of diphenylprolinol silyl ether as an aminocatalyst. As a result, a series of multistereogenic and functionalized spirocyclohexaneoxindoles have been obtained in good yields with moderate diastereoselectivities and excellent enantioselectivities. In addition, electronic circular dichroism (ECD) spectroscopy and time-dependent density functional theory (TD-DFT) were used to investigate the rational structures of spirocyclohexaneoxindoles.