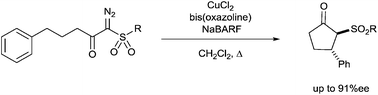
Enantioselectivities in C–H insertion reactions, employing the copper-bis(oxazoline)-NaBARF catalyst system, leading to cyclopentanones are highest with sulfonyl substituents on the carbene carbon, and furthermore, the impact is enhanced by increased steric demand on the sulfonyl substituent (up to 91%ee). Enantioselective intramolecular C–H insertion reactions of α-diazo-β-keto phosphine oxides and 2-diazo-1,3-diketones are reported for the first time.