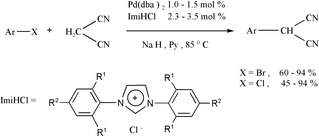
Six imidazolium chlorides (1–6) as precursors of 1,3-diaryl substituted N-heterocyclic carbene ligands were synthesized and evaluated in palladium-catalyzed cross-coupling reactions of aryl chlorides and bromides with malononitrile in the presence of NaH. Among them, 1,3-bis(2,4,6-triethylphenyl)imidazolium chloride (5) and 1,3-bis(2,4,6-triisopropylphenyl)imidazolium chloride (6) are novel. The catalytic system combining Pd(0) with imidazolium salts 4, 5 and 6 with bulky aryl groups in pyridine is found to be superior over others and afforded α-arylmalononitriles in high yields when employing a wide variety of substrates.