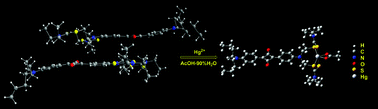
A novel chemosensor based on unsymmetrical squaraine dye (USQ-1) for the selective detection of Hg2+ in aqueous media is described. USQ-1 in combination with metal ions shows dual chromogenic and “turn-on” fluorogenic response selectivity toward Hg2+ as compared to Li+, Na+, K+, Mg2+, Ca2+, Ba2+, Al3+, Cu2+, Cd2+, Mn2+, Fe3+, Ag+, Pb2+, Zn2+, Ni2+ and Co2+ due to the Hg2+-induced deaggregation of the dye molecule. A recognition mechanism based on the binding mode is proposed based on the absorption and fluorescence changes, 1H NMR titration experiments, ESI-MS study, and theoretical calculations.