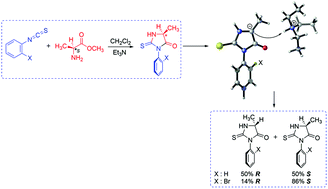
Recently, Sarigul and Dogan have synthesized a number of enantiomerically enriched axially chiral atropoisomeric 2-thiohydantoins by the reaction of L-amino acid ester salts and o-aryl isothiocyanates in the presence of triethyl amine (TEA) in dichloromethane. The non-axially chiral derivative 5-methyl-3-phenyl-2-thiohydantoin gave a racemic product whereas the axially chiral 5-methyl-3-o-bromophenyl-2-thiohydantoin was less prone to racemize at C5 of the heterocyclic ring. In this study, we present a computational study (M06-2X/6-311+G(d,p) for C, H, O, N and S; M06-2X/6-311++G(3df,3pd) for Br) in order to propose plausible mechanisms for the racemization and cyclization steps for 2-thiohydantoin derivatives. The study includes rationalization based on steric as well as the electrostatic effects to elucidate the epimerization differences at C5.