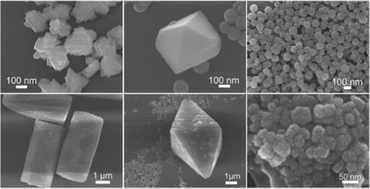
The effect of alkali ions (Li+, Na+, and K+) on controllable synthesis of rare earth (RE) fluorides was investigated by introducing corresponding nitrates into hydrothermal systems. It was found that the products transformed from YF3 truncated octahedra to α-NaYF4 spheres and then to β-NaYF4 microrods as the amount of NaNO3 increased. With the increasing amount, the additive Na+ had the effect on controlling the size of α-NaYF4 particles to decrease. Different alkali ions have distinct abilities of forming corresponding alkali RE fluorides based on their different ionic radii. Possible mechanisms of the alkali cation effect were proposed. By doping Yb3+/Tm3+ ions, upconversion luminescent properties were also investigated.