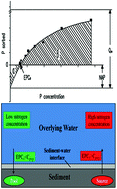
The sorption of phosphorus under different ionic strengths was investigated in four types of sediment samples from the Chinese Dianchi Lake by kinetic and batch equilibrium experiments. The results showed that the sorption rate followed both the pseudo-first order and pseudo-second order. However, the pseudo-second order could better describe the sorption kinetics than the pseudo-first order. Sediments 1 (S1) and 4 (S4) had higher sorption capacities than Sediments 2 (S2) and 3 (S3). The sorption capacities of the four sediments were highly influenced by ionic strength, and low ionic strength was favorable for P uptake. The sorption kinetics of P to sediment was regulated by the surface-diffusion mechanism, and the diffusion rate of P from the liquid–sediment boundary to sediment surface determined its sorption rate. The classic Langmuir isotherm equation was modified to describe P sorption on sediments, and the modified Langmuir isotherm could describe the P sorption well. The native adsorbed phosphorus (NAP), equilibrium phosphorus concentration (EPC0), and maximum phosphorus capacity (Qm) varied with ionic strength. The Fe/Al oxide in the sediment had a significant relationship (R2 > 0.95, P < 0.05) with NAP, EPC0, and Qm. The positions of S1 and S4 had the potential risk of P release, whereas S2 and S3 play a pool role of P.