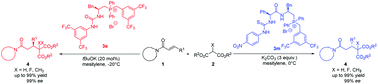
Efficient access to two enantiomers of one chiral compound is critical for the discovery of drugs. However, it is still a challenging problem owing to the difficulty in obtaining two enantiomers of one chiral catalyst. Here, we report a general method to obtain both enantiomeric products via fine tuning the hydrogen-bonding interactions of phosphonium salts. Amino acid derived phosphonium salts and dipeptide derived phosphonium salts exhibited different properties for controlling the transition state, which could efficiently promote the Michael addition reaction to give opposite configurations of products with high yields and enantioselectivities. Preliminary investigations on the mechanism of the reaction and applications of the products were also performed.