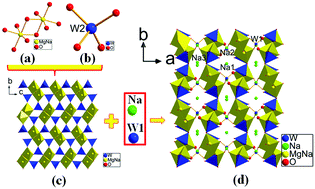
A series of alkali metal magnesium tungstates, A4Mg(WO4)3 (A = Na, K), R2Mg2(WO4)3 (R = Rb, Cs), were synthesized from a high temperature solution, and their structures were determined by single-crystal X-ray diffraction. Interestingly, Na4Mg(WO4)3 crystallizes in the monoclinic space group C2/c, while K4Mg(WO4)3 having an identical stoichiometry with Na4Mg(WO4)3, exhibits a different framework structure belonging to triclinic symmetry with the space group P![[1 with combining macron]](http://www.rsc.org/images/entities/char_0031_0304.gif) . Isostructural Rb2Mg2(WO4)3 and Cs2Mg2(WO4)3 crystallize in the space group P213 of cubic symmetry and reveal a three dimensional framework composed of isolated WO4 tetrahedra, MgO6 octahedra and RO12 (R = Rb, Cs) polyhedra. The effect of the alkali metal cation size on the framework structures of magnesium tungstate has been discussed in detail. In addition, the infrared spectra, as well as the UV-Vis-NIR diffuse reflectance spectroscopy data, are reported. The first-principles theoretical studies are also carried out to aid the understanding of electronic structures and linear optical properties.
. Isostructural Rb2Mg2(WO4)3 and Cs2Mg2(WO4)3 crystallize in the space group P213 of cubic symmetry and reveal a three dimensional framework composed of isolated WO4 tetrahedra, MgO6 octahedra and RO12 (R = Rb, Cs) polyhedra. The effect of the alkali metal cation size on the framework structures of magnesium tungstate has been discussed in detail. In addition, the infrared spectra, as well as the UV-Vis-NIR diffuse reflectance spectroscopy data, are reported. The first-principles theoretical studies are also carried out to aid the understanding of electronic structures and linear optical properties.