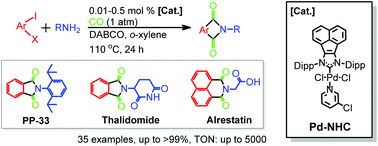
By using a robust acenaphthoimidazolylidene palladium complex (Pd-NHC 1), a scalable approach to access a variety of chiral, pharmaceutical and structurally intriguing N-substituted phthalimides via double aminocarbonylations has been established under atmospheric carbon monoxide pressure at catalyst loadings as low as 0.05 mol%. In addition, the fluorescent properties of the selected N-substituted phthalimide products were also characterized. In comparison with well-known fluorescent molecules, some of them exhibited enhanced violet emission, especially for the ester analogue of Alrestatin, which further confirmed the applicability of the protocol.