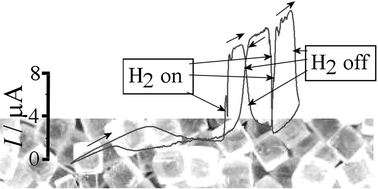
The electrochemical oxidation of formic acid to CO2 is facile at nano-palladium catalysts. In conventional electrochemical systems this process is conducted in aqueous phase and the resulting formation of poorly soluble CO2 gas can limit the kinetics. Here, an alternative electrochemical system with the gas phase in closer contact to the palladium nanoparticle catalyst is investigated based on a glassy carbon electrode and a solid salt electrolyte. It is demonstrated that the reaction zone of salt (here (NH4)2SO4 is most effective), palladium nanoparticle catalyst, and gas phase, is where the electrochemical oxidation process occurs. The effects of the type of salt, the partial pressure of formic acid, and the gas flow rate are investigated. Preliminary data for the oxidation of hydrogen gas at the salt|palladium|electrode contact are reported. A significant salt effect on the palladium catalysed reactions is observed and potential future applications of “salt cells” in sensing are discussed.