In this work, we used finite-field derivative techniques and density functional theory (DFT) to compute the static isotropic polarizability series (α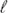 with
with 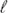 = 1, 2, 3) for the C60–C84 fullerenes and quantitatively assess the intrinsic non-additivity in these fundamental response properties. By comparing against classical models of the fullerenes as conducting spherical shells (or solid spheres) of uniform electron density, a detailed critical analysis of the derived effective scaling laws (α1 ∼ N1.2, α2 ∼ N2.0, α3 ∼ N2.7) demonstrates that the electronic structure of finite-sized fullerenes—a unique dichotomy of electron confinement and delocalization effects due to their quasi-spherical cage-like structures and encapsulated void spaces—simultaneously limits and enhances their quantum mechanical response to electric field perturbations. Corresponding frequency-dependent polarizabilities were obtained by inputting the α
= 1, 2, 3) for the C60–C84 fullerenes and quantitatively assess the intrinsic non-additivity in these fundamental response properties. By comparing against classical models of the fullerenes as conducting spherical shells (or solid spheres) of uniform electron density, a detailed critical analysis of the derived effective scaling laws (α1 ∼ N1.2, α2 ∼ N2.0, α3 ∼ N2.7) demonstrates that the electronic structure of finite-sized fullerenes—a unique dichotomy of electron confinement and delocalization effects due to their quasi-spherical cage-like structures and encapsulated void spaces—simultaneously limits and enhances their quantum mechanical response to electric field perturbations. Corresponding frequency-dependent polarizabilities were obtained by inputting the α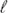 series into the hollow sphere model (within the modified single frequency approximation), and used to compute the molecular dispersion coefficients (Cn with n = 6, 8, 9, 10) needed to describe the non-trivial van der Waals (vdW) interactions in fullerene-based systems. Using first-order perturbation theory in conjunction with >140 000 DFT calculations, we also computed the non-negligible zero-point vibrational contributions to α1 in C60 and C70, thereby enabling a more accurate and direct comparison between theory and experiment for these quintessential nanostructures.
series into the hollow sphere model (within the modified single frequency approximation), and used to compute the molecular dispersion coefficients (Cn with n = 6, 8, 9, 10) needed to describe the non-trivial van der Waals (vdW) interactions in fullerene-based systems. Using first-order perturbation theory in conjunction with >140 000 DFT calculations, we also computed the non-negligible zero-point vibrational contributions to α1 in C60 and C70, thereby enabling a more accurate and direct comparison between theory and experiment for these quintessential nanostructures.

