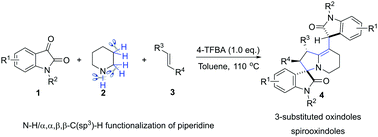
A protocol for the direct functionalization of N–H/α,α,β,β-C(sp3)–H of piperidine without any metal or external oxidants is reported. This reaction is promoted by 4-(trifluoromethyl)benzoic acid via an azomethine ylide intermediate. This is a simple method for the synthesis of spirooxindoles bearing 3-substituted oxindole moieties.