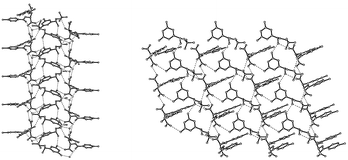
An effective resolving agent, (2S,3S)-di-O-(p-toluoyl) tartaric acid (4), was screened using a ‘family’ approach to yield direct resolution of (R)-terbutaline (1) with high optical purity and yield. Molecular recognition was studied by X-ray crystallographic analyses of the single crystals of the pair of diastereomeric salts. The more-soluble salt formed a sheet supramolecular structure, and the less-soluble salt formed a columnar supramolecular structure by enantiodifferentiating