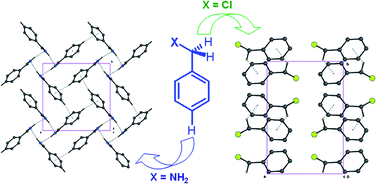
The in situ cryo-crystallization study of benzyl derivatives reveals that the molecular packing in these compounds is either through methylene (sp3)C–H⋯π or aromatic (sp2)C–H⋯π interactions depending on the level of acidity of the benzyl proton. These studies of low melting compounds bring out the subtle features of such weak interactions and point to the directional preferences depending on the nature (electron withdrawing, polarizability) of the neighbouring functional group.