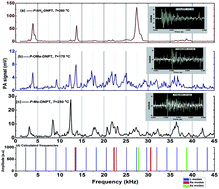
We investigated the effect of the bond lengths of the chemical substituents (attached to the para position of the phenyl ring) on the thermal stability of three newly synthesized nitro rich 1,2,4-triazoles, namely, 1-(4-methyl-3,5-dinitrophenyl)-1H-1,2,4-triazole (P-Me-DNPT), 1-(4-methoxy-3,5-dinitrophenyl)-1H-1,2,4-triazole (P-OMe-DNPT) and 2,6-dinitro-4-(1H-1,2,4-triazol-1-yl)aniline (P-NH2-DNPT). The thermal stability of these compounds was evaluated along with their acoustic fingerprint spectra between 30 and 350 °C, using a pulsed photoacoustic (PA) pyrolysis technique. A 532 nm wavelength of pulse duration 7 ns and repetition rate of 10 Hz, obtained from a Q-switched Nd:YAG laser, was used to determine the released NO2 molecules during the process of thermal decomposition. The thermo gravimetric-differential thermal analysis (TG-DTA) data along with PA results highlight the multistep decomposition mechanism of 1,2,4-triazoles. The study also helps us to distinguish the characteristic behavior of the reported molecules as propellants and explosives for rocket fuels.