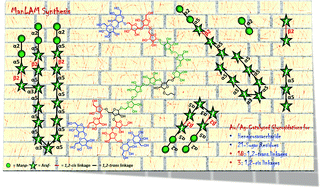
The global incidence of tuberculosis is increasing at an alarming rate, and Mycobacterium tuberculosis (Mtb) is the causative agent for tuberculosis, a disease with high mortality. Lipoarabinomannan (LAM) is one of the major components of the Mtb cellular envelope and is an attractive scaffold for developing anti-tubercular drugs, vaccines and diagnostics. Herein, a highly convergent strategy is developed to synthesize heneicosasaccharyl arabinomannan for the first time. The arabinomannan synthesized in this endeavour has several 1,2-trans or α-Araf linkages and three 1,2-cis or β-Araf linkages end capped with 1,2-trans or α-Manp linkages. All the key glycosidations were performed with alkynyl carbonate glycosyl donors under [Au]/[Ag] catalysis conditions, which gave excellent yields and stereoselectivity even for the reactions between complex and branched oligosaccharides. The resultant allyl oligosaccharide was globally deprotected to obtain the heneicosasaccharyl arabinomannan as a propyl glycoside. In summary, heneicosasaccharyl mannose capped arabinomannan synthesis was achieved in 56 steps with 0.016% overall yield.