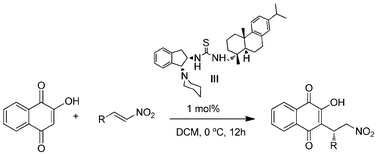
A novel bifunctional rosin-derived indane amine thiourea organocatalyst has been prepared and evaluated its catalytic efficiency in enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinone to β-nitrostyrenes. 3-Alkylnitro-2-hydroxy-1,4-naphthoquinones are obtained remarkably with high enantioselectivities (87–99% ee) and in good yields using low catalyst loading (1 mol%). The catalyst was also found to be effective in catalyzing asymmetric Michael addition of pentane-1,3-dione to trans-β-nitroalkenes.