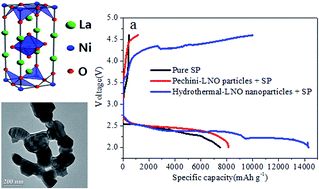
Efficient catalysts for oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) are crucial enabling materials for rechargeable Li–O2 batteries. In the present work, La2NiO4 (LNO) synthesized by a hydrothermal process and modified Pechini method were studied as catalysts for rechargeable Li–O2 batteries. The catalyst prepared by the hydrothermal method shows a smaller particle size and a macroporous structure with 10× higher surface area than that synthesized by the Pechini counterpart, leading to a better electrocatalytic activity. The improved OER catalytic activity of the hydrothermal-LNO nanoparticles was confirmed by a 150 mV lower recharge potential than the Pechini-LNO particles and catalyst-free pure Super P (SP) electrode. In addition, the hydrothermal-LNO catalyzed battery cell delivered a first discharge capacity of 14 310.9 mA h g−1 at 0.16 mA cm−2, compared to 8132.4 mA h g−1 of the Pechini-LNO and 7478.8 mA h g−1 of the pure SP electrode, demonstrating higher catalytic ORR activity of the hydrothermal-LNO particles. Overall, the LNO nanoparticles are a promising cathode catalyst for non-aqueous electrolyte based Li–O2 batteries.