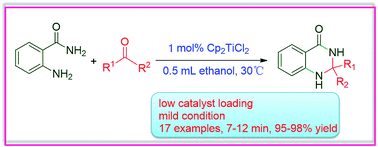
An efficient catalytic system involving in situ activation of kinetically inert titanocene dichloride with alcoholic solvent for the synthesis of quinazoline derivatives was developed. 1 mol% Cp2TiCl2 at 30 °C afforded 17 examples of quinazoline derivatives with yields of 95–98% in 7–12 minutes. Mechanistic experiments using in situ NMR and HRMS established that the coordination of ethanol to the titanocene moiety released the catalytic species [Cp2Ti(OCH2CH3)2].