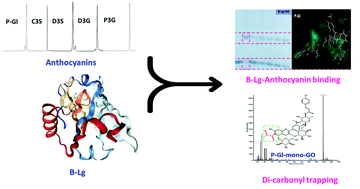
We elucidated the underlying mechanisms of the anti-glycoxidation effects of five structurally different anthocyanins on glycated-β-lactoglobulin (β-Lg). The results indicated that anthocyanins structurally inhibited the formation of advanced glycation end-products, where petunidin-3-rutinoside-(p-coumaryl)-5-glucoside (Pt-Gl) exerted higher effects than those of others (p < 0.05). Through the three main steps of glycoxidation, anthocyanins trapped intermediate dicarbonyls and blocked some of the glycation sites of β-Lg. UPLC-ESI-Q-TOF-MS characterized that these anthocyanins structurally formed mono- and di-GO/MGO adducts, and Pt-Gl formed adducts with both dicarbonyls. More importantly, Pt-Gl interacted with some of the glycation sites of β-Lg such as Lys100, Lys101, and Arg124. Structurally, it was found that high–molecular weight anthocyanins with coumaric acid acylation seem to be better than others, which was followed by di- and mono-glycoside anthocyanins. Overall, GO/MGO-trapping and β-Lg-anthocyanin binding are revealed as the key mechanisms of the anti-glycoxidation effects of anthocyanins on β-Lg, which could be used as effective glycation inhibitors in protein-rich matrices.