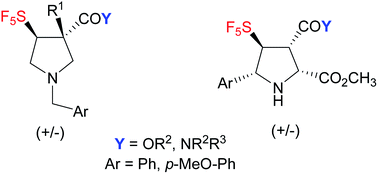
For the first time, di-, tri- and tetrasubstituted pentafluorosulfanylated pyrrolidines have been efficiently synthetized via 1,3-dipolar cycloaddition. In the case of tetra-substituted pyrrolidines, an unusual mixture of 1/1 regioisomers was obtained. Theoretical calculations have been carried out and the regioselectivity has been explained compared to the results previously obtained in the trifluoromethylated pyrrolidine series.