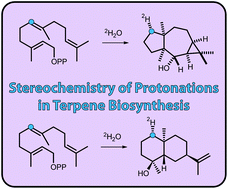
Three sesquiterpene cyclases from Streptomyces scabei 87.22, Streptomyces venezuelae ATCC 10712 and Streptomyces clavuligerus ATCC 27064 were characterised and their products were identified as (−)-neomeranol B, (+)-isodauc-8-en-11-ol and (+)-intermedeol, respectively. The stereochemical courses of the terpene cyclisations were investigated by use of various 13C-labelled FPP isotopomers. A quick and easy test was developed that allows to distinguish reprotonations of olefinic double bonds in neutral intermediates from the two stereoheterotopic faces. The method makes use of incubating 13C-FPP isotopomers labelled at the reprotonated carbon in deuterium oxide and subsequent HSQC analysis of the product. A 1,7-cyclisation towards (+)-isodauc-8-en-11-ol was followed by use of (1,7-13C2)FPP. Surprisingly, the (+)-isodauc-8-en-11-ol also accepted (2Z,6E)-FPP resulting in the same product profile as obtained from (2E,6E)-FPP.