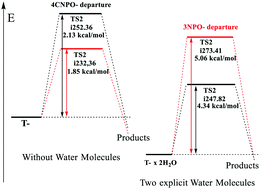
The reactions of O-(4-cyanophenyl) O-(3-nitrophenyl) thionocarbonate (1) with morpholine and aniline lead to the formation of 4-cyanophenol and 3-nitrophenol in a 1.5 : 1 ratio. This ratio corresponds to a δΔG± value of about 250 cal mol−1 for the expulsion of both nucleofuges. These reactions follow a mechanism through two tetrahedral intermediates: one zwitterionic (T±) and other anionic (T−). As a model, the reaction of 1 with morpholine was also theoretically examined using DFT methods. Theoretical calculations in the gas phase predict a distribution of 4-cyanophenol : 3-nitrophenol near 1 : 1.12, which differs from the experimental result. However, the microsolvation by two explicit water molecules predicts a product distribution of 1.34 : 1. Theoretical results show the dependency of the nucleofugality ratio when the reaction is assisted by water molecules. The results indicate that the energy barriers are mainly affected by kinetic variables more than the thermodynamic ones.