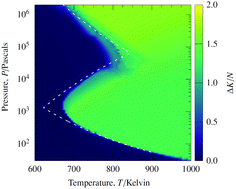
The explosion limits of hydrogen–oxygen mixtures are macroscopic, temperature–pressure boundaries that divide the overall chemistry of hydrogen oxidation into slow-burning and explosive regimes. Here, we demonstrate that it is possible to recover the three chemical explosion limits of H2/O2 mixtures from nonequilibrium stochastic trajectories. This demonstration relies on the finding that, in explosive regimes, these trajectories have the quantitative features of a dynamical phase transition. Through computer simulations for both a generic and a reduced model for hydrogen oxidation, we find only one dominant reactive phase at temperatures below the explosion limits. At temperatures above the limits, however, a second phase transiently emerges from the chemistry. By locating the pseudo-critical temperature where two reactive phases are distinguishable, we construct all three explosion-limit boundaries for model hydrogen–oxygen mixtures of finite size.