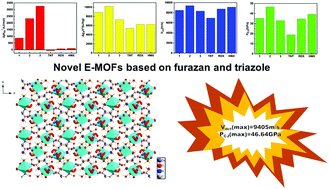
Energetic metal–organic frameworks (EMOFs) have witnessed increasing development and been proved as promising candidates for new high energy density materials (HEDMs). Here, three metal energetic complexes based on 3,4-diaminofurazan (DAF) and 3-amino-1H-1,2,4-triazole (Hatz) ligands have been synthesized via a routine method and fully characterized by IR, 1H NMR, 13C NMR spectroscopy, X-ray single-crystal diffraction and DSC-TG techniques. The standard heats of combustion were obtained by an oxygen bomb calorimeter, and the detonation performance was calculated using the EXPLO5 program. Crystal structure determinations reveal that 1 shows a one-dimensional zigzag chain structure consisting of Zn(II) and bidentate DAF, ultimately producing a 2D MOF through extensive hydrogen bond interactions. The crystal densities of these complexes are up to 1.9 g cm−3 at 298 K. The thermal analyses from the DSC-TG test show that most of them have good thermal stability with the highest decomposition temperature of 241 °C. The as-synthesized compounds possess highly positive heats of formation with values for 2 and 3 of 2329.30 kJ mol−1 and 3261.57 kJ mol−1, respectively, and high heats of detonation with value for 2 of 10231 kJ kg−1, superior to that of HMX and RDX, which are most powerful organic explosives in use today. Expectedly, they exhibit excellent detonation performance, of which 2 has the best detonation velocity (9405 m s−1) and detonation pressure (46.64 GPa), and thus shows a great promise for potential applications as a high-energy density material. In addition, DFT calculations were performed in order to investigate the electronic properties based on the structures. This study highlights the great potential of nitrogen-rich group containing EMOFs (or complexes) for new eco-friendly energetic materials.