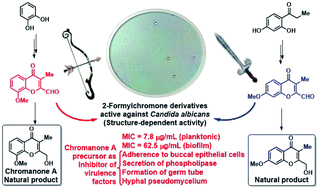
A straightforward and convenient approach for the first total syntheses of chromanone A and a related 7-OMe substituted natural product is reported. These unique C-3 substituted 2-hydroxymethyl chromones were recently isolated as fungal metabolites. Chromanone A was synthesized in 25.3% overall yield from the readily available pyrocatechol, whereas the second natural product was prepared in 39.7% global yield. A small library of chromones, including both natural products and some of their synthetic heterocyclic precursors, was evaluated against Candida albicans ATCC 10231, a biofilm forming agent. It was found that 8-methoxy-3-methyl-4-oxo-4H-chromene-2-carbaldehyde, a partially oxidized form of chromanone A, exhibited a minimum inhibitory concentration of 7.8 μg mL−1 and significantly inhibited the yeast's virulence factors, including the adherence to buccal epithelial cells and the secretion of phospholipases, as well as the formation of germ tubes and the generation of the hyphal pseudomycelium. In addition, despite the heterocycle exhibiting non-significant inhibition of the formation of the Candida biofilm, it completely inhibited the growth of C. albicans in preformed biofilms at 62.5 μg mL−1.