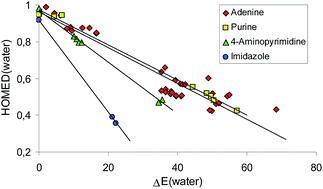
Heterocycles containing one or more amidine moieties {–NH–C(R) ![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N–} such as adenine and its building blocks, imidazole, 4-aminopyrimidine, and purine, are excellent examples of tautomeric systems for which changes of position(s) of labile proton(s) cause parallel changes of geometric and energetic parameters for prototropic tautomers. One-electron oxidation has a slight effect on this relationship. The amino-imine conversions within the amidine group(s) are favored. Well delocalized (aromatic) tautomers, containing labile proton(s) at heteroatom(s), are major, minor, or rare forms. Non-aromatic tautomers with a labile proton at the C atom can be considered as very rare isomers. Dramatic changes take place for reduced heterocycles. Electron delocalization is not the main factor that dictates tautomeric preferences. The HOMED/ΔE relationship seems to be more complex. The enamino-imine conversions predominate and some very rare forms have the lowest energies. This clearly shows the importance of very rare tautomers, often neglected in proton-transfer, electron-transfer, and ion–radical reactions.
N–} such as adenine and its building blocks, imidazole, 4-aminopyrimidine, and purine, are excellent examples of tautomeric systems for which changes of position(s) of labile proton(s) cause parallel changes of geometric and energetic parameters for prototropic tautomers. One-electron oxidation has a slight effect on this relationship. The amino-imine conversions within the amidine group(s) are favored. Well delocalized (aromatic) tautomers, containing labile proton(s) at heteroatom(s), are major, minor, or rare forms. Non-aromatic tautomers with a labile proton at the C atom can be considered as very rare isomers. Dramatic changes take place for reduced heterocycles. Electron delocalization is not the main factor that dictates tautomeric preferences. The HOMED/ΔE relationship seems to be more complex. The enamino-imine conversions predominate and some very rare forms have the lowest energies. This clearly shows the importance of very rare tautomers, often neglected in proton-transfer, electron-transfer, and ion–radical reactions.