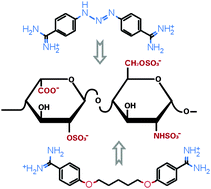
It is shown that the antiprotozoal drugs berenil and pentamidine, conventional minor groove binders of DNA, form non-covalent complexes with polyanionic glycosaminoglycans. Induced circular dichroism (CD) spectra as well as UV hypochromism confirmed drug binding to the asymmetric template of heparin and chondroitin 6-sulfate. The biphasic nature of the CD signals refers to intermolecular chiral exciton coupling between the dicationic guest molecules forming a right- or a left-handed helical array along the GAG chains. Quantitative evaluation of the spectroscopic data measured in pH 7.0 buffer solution (80 mM NaCl) indicated a higher (Ka ∼ 106 M−1 for berenil) and a lower (Ka ∼ 105 M−1 for pentamidine) affinity heparin binding of these agents, similar to that reported for DNA. Drug–chondroitin sulfate complexes (Ka ∼ 104–105 M−1) could be detected only at low ionic strength. These results imply that besides nucleic acids, GAGs may be another pharmacological targets for diarylamidine drugs.