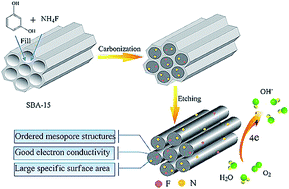
Fluorine and nitrogen co-doped ordered mesoporous carbon (FN-OMC) is synthesized by a hard template method employing inexpensive resorcin as a carbon precursor and ammonium fluoride as a fluorine and nitrogen source. Considerable fluorine and nitrogen atoms (F: 0.35 at%, N: 2.25 at%) are homogeneously implanted in the graphitic carbon matrix with high surface area (671.3 cm2 g−1) and ordered mesoporous channels (6.2 nm). The FN-OMC prepared at the optimized heat-treatment temperature (1000 °C) exhibits a high catalytic activity (Eonset: −0.07 V, E1/2: −0.19 V, current density: 5.83 mA cm−2) for oxygen reduction reaction (ORR) comparable to commercial platinum–carbon (Pt–C, 20 wt%) catalysts, but better stability and methanol-tolerance in alkaline solutions. The synergistic effect of fluorine and nitrogen atoms in carbon frameworks is demonstrated to sharply polarize adjacent carbon atoms and facilitate the formation of defect-induced active sites for ORR.