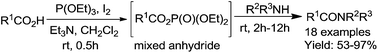With the activation of P(OEt)3 and I2, carboxylic acids can smoothly react with various primary and secondary amines, affording a series of amides, including peptides without racemization. 31P NMR spectroscopy studies showed that carboxylic phosphoric mixed anhydride was the reactive intermediate and a possible mechanism was herein proposed.