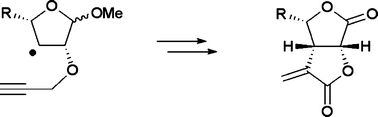
Radical cyclization reactions were performed by 5-exo-dig mode to yield cis-fused bicyclic systems, leading to the synthesis of bis-butyrolactone class of natural products. The study was aimed at understanding the impact of alkyl side chains of furanoside ring systems in L-ara configuration on the radical cyclization. It was amply demonstrated by experimental studies that the increase in the length of the alkyl side chain has an effect on the cyclization: while efficient cyclization reactions could be realized with methyl and ethyl side chains, the yields were significantly reduced in the case of n-pentyl side chain. Theoretical studies using DFT and (RO)MP2 methods were carried out to analyze the influence of the substitution pattern on the cyclization barriers.