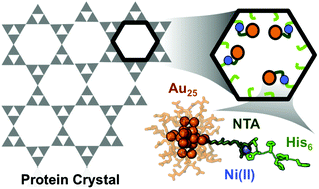
DNA assemblies have been used to organize inorganic nanoparticles into 3D arrays, with emergent properties arising as a result of nanoparticle spacing and geometry. We report here the use of engineered protein crystals as an alternative approach to biologically mediated assembly of inorganic nanoparticles. The protein crystal's 13 nm diameter pores result in an 80% solvent content and display hexahistidine sequences on their interior. The hexahistidine sequence captures Au25(glutathione)∼17 (nitrilotriacetic acid)∼1 nanoclusters throughout a chemically crosslinked crystal via the coordination of Ni(II) to both the cluster and the protein. Nanoparticle loading was validated by confocal microscopy and elemental analysis. The nanoparticles may be released from the crystal by exposure to EDTA, which chelates the Ni(II) and breaks the specific protein/nanoparticle interaction. The integrity of the protein crystals after crosslinking and nanoparticle capture was confirmed by single crystal X-ray crystallography.