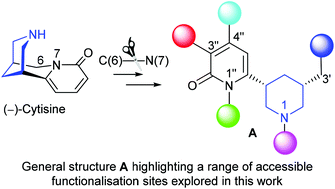
N-Benzyl cytisine undergoes an efficient C(6)–N(7) cleavage via directed C(6) lithiation, borylation and oxidation to provide a “privileged” heterocyclic core unit comprising a highly functionalised, cis-3,5-disubstituted piperidine in enantiomerically pure form. The potential offered by this unit as a means to explore chemical space has been evaluated and methods have been defined (and illustrated) that allow for selective manipulation of N(1), C(3′), and the pyridone N. The pyridone core can also be diversified via bromination (at C(3′′) and C(5′′)) which is complementary to direct C–H activation based on Ir-catalyzed borylation to provide access to C(4′′). The use of a boronate-based 1,2-migration as an alternative trigger to mediate C(6)–N(7) cleavage of cytisine was evaluated but failed. However, the stability of the intermediate boronate opens a new pathway for the elaboration of cytisine itself using both Matteson homologation and Zweifel olefination.