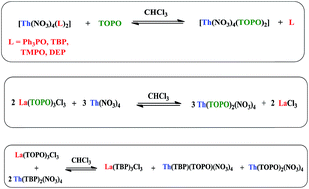
Some selected aminophosphine oxides (AmPOs) of the type OP(NMe2)3, OPPh(NMe2)2, OP(NC2H4O)3, OPPh(NC2H4O)2 and their corresponding La(III) and Th(IV) complexes are synthesized and analyzed by FT-IR, 1H-NMR, 31P{1H}-NMR, elemental analysis and TGA data. The coordination behavior of AmPOs was compared with some of the known ligands that include trioctylphosphine oxide (TOPO), tributylphosphate (TBP) and diethylphosphite (DEP). Thermogravimetric analysis of these complexes showed a distinct decomposition trend either by a single step or multi-step elimination of ligand species, which are strongly dependent on the electronic and steric behaviour of substituents on the P![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O group and the nature of the metal. Phosphine oxide based La(III) and Th(IV) complexes undergo three unique intermolecular ligand exchange reactions at room temperature: relative competition among phosphine oxides to form a strong complex by exchanging the weaker ligand and complete ligand transfer from La(III) to Th(IV) metal centers. Ligand crossover is well controlled by priority rules and the trend is TOPO > TBP > DEP > AmPO > Ph3PO. This tendency closely agrees with the stability constants of metal complexes. On comparison, Th(IV) complexes showed slightly higher stability than La(III) analogues.
O group and the nature of the metal. Phosphine oxide based La(III) and Th(IV) complexes undergo three unique intermolecular ligand exchange reactions at room temperature: relative competition among phosphine oxides to form a strong complex by exchanging the weaker ligand and complete ligand transfer from La(III) to Th(IV) metal centers. Ligand crossover is well controlled by priority rules and the trend is TOPO > TBP > DEP > AmPO > Ph3PO. This tendency closely agrees with the stability constants of metal complexes. On comparison, Th(IV) complexes showed slightly higher stability than La(III) analogues.