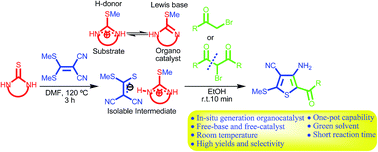
An organic salt generated by cyclic thioureas and 2-di(methylsulfanyl)methylene malononitrile in reaction with primary and secondary α-haloketones leads to tetrasubstituted thiophenes without using additional base or catalyst at room temperature. The S-methylisothiourea moiety of this salt as an organocatalyst performs a hybrid function of thiourea and imidazole to activate electrophiles via H-bonding and trigger cyclization via Lewis basicity character, respectively. Interestingly, a green solvent (in two step mode), short reaction time, easy purification, high yield and selectivity accompany this protocol. The structures of the intermediate and afforded adducts are fully characterized by analytical investigations and X-ray crystallography.