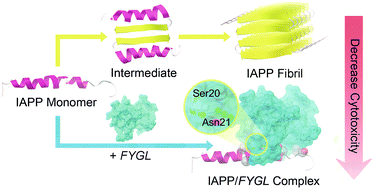
The self-association of human islet amyloid polypeptide (IAPP) into cytotoxic fibrillar assemblies that induce pancreatic β-cell apoptosis has been postulated as one of the major contributors to the development of type 2 diabetes (T2D). Hence, preventing IAPP amyloid fibrillation and β-cell apoptosis is regarded as a promising treatment for T2D. Intrigued by our previous findings that a natural amphiphilic hyperbranched proteoglycan from Ganoderma lucidum, named FYGL, can decrease plasma glucose level in vivo, we demonstrated here that FYGL efficiently inhibits IAPP fibrillation and attenuates β-cell apoptosis, using a combination of biophysical, calculation and cell culture methods, including thioflavin T (ThT) and 8-anilino-1-naphthalene sulfonate (ANS) fluorescence spectroscopy, transmission electron microscopy (TEM), atomic force microscopy (AFM), circular dichroism (CD) spectroscopy, dynamic light scattering (DLS), nuclear magnetic resonance (NMR) spectroscopy, molecular docking, cell viability tests and laser scanning confocal microscopy imaging. Moreover, a universally applicable inhibition mechanism proposed that FYGL blocks the interpeptide interaction and stabilizes IAPP structure in an α-helical conformation by forming multiple hydrogen bonds with IAPP. The findings of this study warrant FYGL to be further investigated as a potential therapeutic treatment and may provide a valuable reference for medicinal chemistry in the development of drugs against T2D as well as other amyloid diseases such as Alzheimer's disease and Huntington's disease.