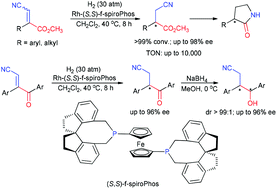
A highly efficient and enantioselective synthesis of γ-lactams and γ-amino acids by Rh-catalyzed asymmetric hydrogenation has been developed. Using the Rh-(S,S)-f-spiroPhos complex, under mild conditions a wide range of 3-cyano acrylate esters including both E and Z-isomers and β-cyano-α-aryl-α,β-unsaturated ketones were first hydrogenated with excellent enantioselectivities (up to 98% ee) and high turnover numbers (TON up to 10 000).