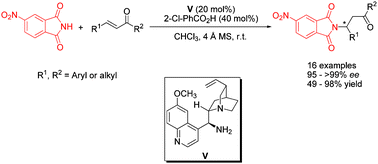
The first highly enantioselective aza-Michael addition reactions of 4-nitrophthalimide with α,β-unsaturated ketones have been reported. The reactions gave the corresponding Michael adducts in moderate to good yields (49–98%) and excellent enantioselectivities (95–>99% ee). Computational studies demonstrate that the reason why 4-nitrophthalimide acts as a suitable nucleophile may be because it is more easily activated and therefore it has a stronger nucleophilicity.