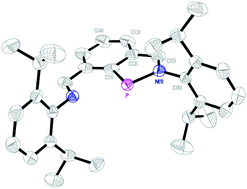
Diiminophenyl (dimph) proved to be an excellent ligand platform to stabilise a low-valent phosphorus centre. The resultant compound dimphP (3), which can be rationalized as an imino-stabilised phosphinidene or benzoazaphosphole, shows remarkable chemical stability: it withstands treatment with water and oxidizers (O2 and pyridine oxide) and only reacts with excess strong acid (e.g. HCl) to generate the P(III) chloride (dimHph)PCl (7), where dimHph stands for a diiminophenyl group hydrogenated at one imine position. Surprisingly, substitution of the chloride under some nucleophilic (KOBut) and electrophilic conditions (Me3SiOTf) regenerates the parent compound 3, by proton removal from the weakly acidic CH2N position. A related species (dimH2ph)P (14), where dimH2ph stands for diiminophenyl hydrogenated to imino(aminomethyl)phenyl, is produced upon thermal rearrangement of the hydride (dimHph)PH (13). X-ray structures of compounds 3, 14, and (dimHph)PO2CH (17) are also discussed.