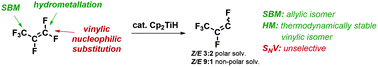Catalytic hydrodefluorination of perfluoroallylbenzene with Cp2TiH in THF is unselective and yields a variety of previously unknown compounds, predominantly activated in the allylic position. Several different mechanisms have been examined in detail using solvent corrected (THF) DFT(M06-2X) calculations for the archetypal perfluorinated olefin perfluoropropene and perfluoroallylbenzene: (a) single electron transfer, (b) hydrometallation/fluoride elimination, (c) σ-bond metathesis (allylic or vinylic), and (d) nucleophilic vinylic substitution (SNV, w/o Ti–F contacts in the TS). SNV is shown to be a competitive mechanism to hydrometallation and proceeds via ionic species from which F-elimination is facile and unselective leading to low selectivity in polar solvents. Subsequent experiments show that selectivity can be increased in a non-polar solvent.