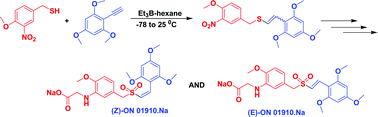
Hydrothiolation of benzyl mercaptan to arylacetylene: application to the synthesis of (E) and (Z)-isomers of ON 01910·Na (Rigosertib®), a phase III clinical stage anti-cancer agent†
Venkat R. Pallela,Muralidhar R. Mallireddigari,Stephen C. Cosenza,Balaiah Akula,D. R. C. Venkata Subbaiah,E. Premkumar Reddy,M. V. Ramana Reddy
Abstract
A stereoselective and efficient method for free radical addition of benzyl thiol to aryl acetylene in the presence of Et3B-hexane has been developed for the synthesis of (Z) and (E)-styryl benzyl sulfides where base catalyzed hydrothiolations have failed. The scope of this reaction was successfully extended for the synthesis of (E)-ON 01910·Na, a phase III clinical stage anti-cancer agent and its inactive geometrical isomer (Z)-ON 01910·Na. It is interesting to note that all the E-isomers synthesized have shown better cytotoxicity profile on cancer cells compared to the Z-isomers.