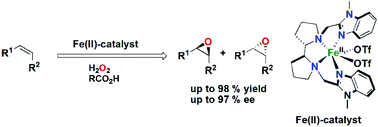
The chiral tetradentate N4-donor ligand, 1-methyl-2-({(S)-2-[(S)-1-(1-methylbenzimidazol-2-yl methyl)pyrrolidin-2-yl]pyrrolidin-1-yl}methyl) benzimidazole (S,S-PDBzL), based on a chiral dipyrrolidine backbone, has been synthesized and its corresponding Fe(II) complex has been prepared and characterized. The X-ray structure of the complex reveals that the Fe(II) ion is in a distorted octahedral coordination environment with two cis-oriented coordination sites occupied by (labile) triflate anions. The ability of the iron complex to catalyze asymmetric epoxidation reactions of olefins with H2O2 was investigated, using 2-cyclohexen-1-one, 2-cyclopenten-1-one, cis-β-methylstyrene, isophorone, chalcones and tetralones as substrates. Different carboxylic acids were used as additives to enhance yields and enantioselectivities, and 2-ethylhexanoic acid was found to give the best results. The catalysis results indicate that the Fe(II) complex is capable of effecting comparatively high enantioselectivities (>80%) in the epoxidation reactions.