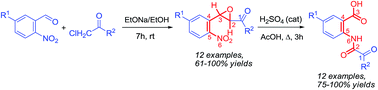
A novel one-pot synthetic approach to N1-(2-carboxyaryl)-N2-(aryl or H)oxalamides from 3-(2-nitroaryl)oxirane-2-carboxamides via the classical Meinwald rearrangement and a new rearrangement sequence has been developed. The methodology is applicable to the synthesis of N-(2-carboxyphenyl)aryloxalmonoamides from (3-(2-nitrophenyl)oxiran-2-yl)(aryl)methanones. The method is operationally simple and high yielding, thus providing a new useful formula for both anthranilic acid derivatives and oxalamides.