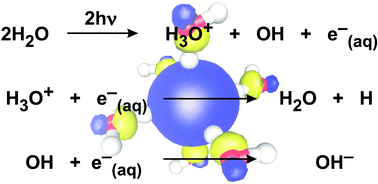
Hydrated electrons were prepared by multi-photon ionization of neat water with 266 nm light. Using femtosecond pump–probe spectroscopy the dynamics of geminate recombination of the solvated electrons were studied over a wide temperature (296 K ≤ T ≤ 660 K) and density (0.18 g cm−3 ≤ ρ ≤ 1.00 g cm−3) range extending from the liquid well into the supercritical phase of water. The probability that hydrated electrons escape an initial recombination was found to strongly decrease with increasing temperature. In contrast, the isothermal density-dependence of this survival probability above the critical temperature was surprisingly weak. The peculiar dependence of the initial electron annihilation process on the thermodynamic state variables is discussed in terms of the Onsager model for initial recombination of ion pairs and an effective shielding of the electrostatic interactions of the recombining partners. A finite escape probability for a dielectric constant approaching unity can be interpreted by the existence of a minor fraction of highly mobile electrons created via autoionization.