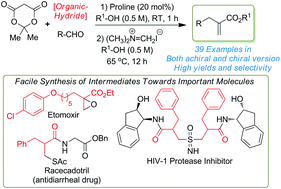
A general process for the high-yielding synthesis of substituted chiral and achiral α-substituted acrylates was achieved through the sequential one-pot combination of a metal-free reductive coupling reaction followed by an Eschenmoser methylenation. The proline catalyzed reaction of Meldrum's acid, aldehydes and Hantzsch ester followed by methylenation was successful with Eschenmoser's salt in the presence of an alcohol solvent. Herein, we have shown the high-yielding synthesis of privileged building blocks from chiral/achiral α-substituted acrylates and shown them to be very good intermediates in the pharmaceuticals and natural products synthesis.