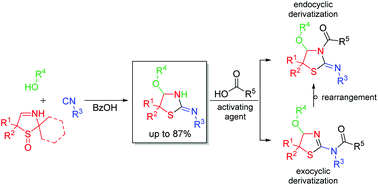
An unexpected formation of cyclic α-alkoxy isothioureas has been achieved. As is known, the heterocyclic imines 2,5-dihydro-1,3-thiazoles are convertible to bisamides with the aid of a carboxylic acid and an isocyanide (Ugi reaction). Herein, it is shown that 2,5-dihydro-1,3-thiazole S-monoxides—the respective α-sulfinyl imines—are characterized by an altered reaction behavior. In a hitherto unknown multicomponent reaction the α-sulfinyl imines react with an isocyanide under acidic conditions in an alcoholic solution to the respective α-alkoxy isothioureas in good yields. In addition to the investigations on this unexpected synthesis the regioselectivity of the acylation of the synthesized compounds is described. A rearrangement, which is accelerated by EDC and HOBt, between both possible regioisomers was found.