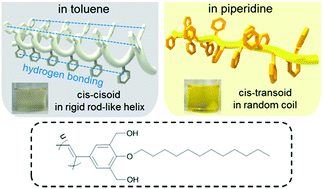
The chain rigidity, liquid crystallinity, absorptivity, and photoluminescence of a helical poly(phenylacetylene) derivative varied significantly depending on the solvent employed, owing to the conformational changes caused by the formation or destruction of intramolecular hydrogen bonds. The polymer chains were rigid and liquid crystalline when dissolved in such non-solvents as toluene and THF to show lower absorptivity and intramolecular excimer emission while the same polymer chains became flexible in piperidine having a high hydrogen-bonding acceptor strength to exhibit strong absorption in the visible region and the emission was significantly quenched.