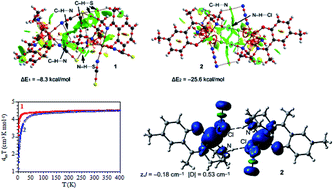
The crystal structure and magnetic properties of two mononuclear iron(III) Schiff base complexes, [FeL1(NCS)2] (1), HL1 = 2-[1-[[2-[(2-aminoethyl)amino]ethyl]imino]ethyl]phenol and [FeL2(N3)Cl] (2), HL2 = 2-(-1-(2-(2-aminoethylamino)ethylimino)ethyl)-4-methylphenol are reported. Each complex contains a Fe(III) ion surrounded by a N3O Schiff base ligand and two NCS− ligands (in 1) or one N3− and one Cl− ligands (in 2). The magnetic properties can be well reproduced with zero field splittings in the high spin S = 5/2 Fe(III) ions and weak intermolecular Fe–Fe interactions mediated by hydrogen bonds. This intermolecular antiferromagnetic interaction has been validated by using DFT calculations in complex 2. Moreover, the interaction energies of the H-bonded dimers in both complexes have been estimated using DFT calculations and characterized using a combination of QTAIM and NCI plot computational tools. Complexes 1 and 2 constitute two rare examples of Fe(III) complexes with magnetic interactions through H-bonds.