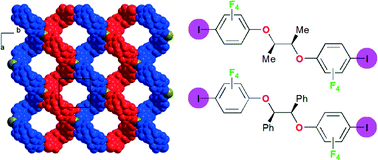
Chiral, ditopic, halogen bond donor molecules are prepared from the reaction of C6F5I with three different enantiopure chiral diols, namely, (R,R)-2,3-butanediol, (R,R)-hydrobenzoin and S-binaphthol, with displacement of the fluorine atom para to the iodine atom in C6F5I, to give (R,R)-1, (R,R)-2 and (S)-3, respectively. Chiral, halogen-bonded networks with halide anions are investigated upon co-crystallisation of (R,R)-1 with (Et3S+, I−), (Et4N+, Br−), (n-Bu4N+, Br−) and (n-Pe4N+, Br−). In the first three salts with 1 : 1 stoichiometry, the halide anions are coordinated by two iodine atoms with short I⋯X− (X− = I−, Br−) distances and acute (75–80°) I⋯X−⋯I angles, leading to the formation of chains, eventually organized into helices around twofold screw axes as in [(R,R)-1]·[Bu4NBr]. Co-crystallisation with tetrapentylammonium bromide affords a 2 : 1 stoichiometry salt, [(R,R)-1]2·[Pe4NBr], with a fourfold coordination around the Br− anion and the formation of a square lattice network built out of interconnected helices.